|
|
|
In order to produce high quality in vivo images using
fluorescence imaging technologies, it is important to have
as low background signal as possible. It has been shown
that laboratory animal diets containing chlorophyll fluoresce
at 680 nm, which can interfere with the imaging of many
common in vivo fluorophores such as GFP or Alexafluor
650 and 680. The confounding fluorescent signal they
produce as they pass through the gastrointestinal tract
makes quantification of true signal difficult. It appears that
unrefined chlorophyll-containing ingredients, particularly
alfalfa, are responsible for this ‘noise’. |
|
|
|
Chow
The ingredients used to make lab animal chows contain both
nutritive and non-nutritive components, both of which will vary
with season and harvest location. Because of these differences
over time, chow companies use what is sometimes called the
“constant nutrition” method of formulation which entails altering
the concentration of each ingredient to ensure constant
macro nutrient content from batch to batch. However, in
changing the levels of alfalfa for example, the amounts of non-
nutritive compounds which “ride along” with the alfalfa are also
changed. These compounds can include heavy metals such as
arsenic, compounds used in pesticides, and phytoestrogens.
Many of these compounds have been shown to have an effect
at the molecular level of gene expression. Phytoestrogens have
been shown to inhibit atherosclerosis, hypertension, obesity,
and diabetes and the formation of some cancers. One is forced
to acknowledge that some of the plant-based ingredients in
chows contain biologically active compounds which can affect
the phenotype of the animal and furthermore, the levels of these
compounds will likely vary from batch to batch.
OpenSource Diets
Purified ingredient, OpenSource Diets are formulated and
manufactured using very highly refined, chlorophyll-free
ingredients. While the lack of chlorophyll in OpenSource diets
provides convincing evidence for their use for in vivo imaging
studies, the very nature of OpenSource purified diets argues for
their use in all lab animal research.
The highly refined ingredients used in OpenSource diets mean
minimal batch-to-batch variation, reducing data variability due
to diet. Secondly, the fact that each ingredient contains one
nutrient makes it relatively simple to change the nutrient
content of a diet to meet the needs of the researcher. Lastly,
the known nutritional content and lack of variability in
OpenSource purified diets means that researchers around the
world can reliably report and repeat their studies.
Study-specific OpenSource Diets can be formulated in
consultation with scientists in the Research Diets Resource
Center. By using data from the scientific literature and our own
25 years of experience in this industry, we can provide
researchers with information they need to decide which diets
may be the most appropriate for their study.
Contact our Resource Center at info@researchdiets.com to
discuss your OpenSource Diet needs.
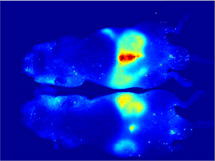
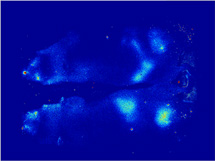
Clean Background
OpenSource Diets provide minimal background
autofluorescence
when conducting in vivo imaging studies in
mice. The time
required for a diet to clear the intestinal tract
when changing
from a standard mouse chow, high in
autofluorescence, to a
purified diet is illustrated with the use of
the Pearl Imager
(LI-COR Biosciences). Red signal represents
the 700 nm
autofluorescence due to the standard mouse chow
containing
plant material. The animal received IRDye 800CW
BoneTag™
(LI-COR Biosciences) to label skeletal features for
easy
identification of the abdominal region (grayscale
represents
800 nm signal).
|
|
Photo courtesy of CRi 2 |
| |
|
|
|
|
The MaestroTM in vivo imaging
system is an LCTF - based
multispectral imaging system which can capture reflectance and fluorescence images of small animals at multiple wavelengths. Spectral analysis software can then “unmix” multiple signals, remove autofluorescence contributions and greatly increase sensitivity and quantitative accuracy .1
1) Richard M. Levenson and James R. Mansfield, “Spectral Imaging in Biology and Medicine: Slices of Life” Cytometry
A . 2006 Aug; 69(8):748-58.
2) Matthew B. Bouchard, Sarah A. MacLaurin, Peter J. Dwyer, James Mansfield, Richard Levenson, and Thomas Krucker " Technical Considerations in Longitudinal Multispectral Small Animal Molecular Imaging" Journal of Biomedical Optics ,
in press. 3) Matthew B. Bouchard, Sarah A. MacLaurin, Peter J. Dwyer, James Mansfield, Richard Levenson, and Thomas Krucke," Reduction of Skin and Food Autofluorescence in
Different Mouse Strains through Diet Changes". Poster 2006 Joint Molecular Imaging Conference. |